Hierarchical Analysis of Multidrug-Resistant Organism Clearance and Asymmetric Co-Colonization in a Korean Long-Term Care Facility (Frank et al., Accepted October 19, 2025)
Hyunsuk Frank Roh, MD; Dong Kwon Shin, RN; Do-Yeon Kim, RN; and Jung Mogg Kim, MD, PhD*
Highlights
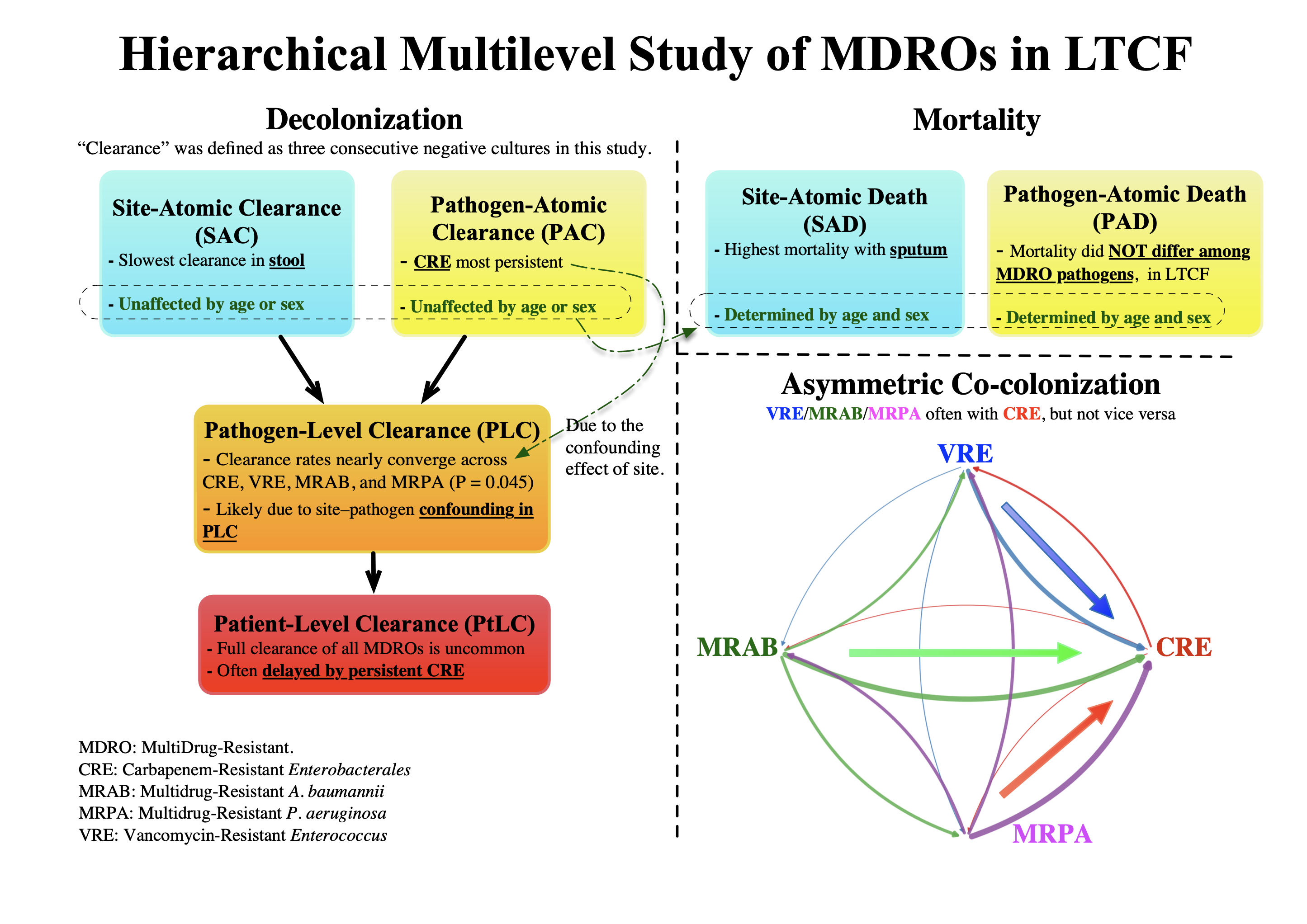
- Four-tier analysis separates site and pathogen confounding effects in decolonization
- Sputum carriage and patient factors—not pathogen—drive mortality, in LTCF
- Stool and CRE decolonize slowest, unexpectedly independent of patient age or sex
- Asymmetric co-colonization: non-CRE often with CRE, but not vice versa → screen for CRE after non-CRE isolation
- Investigate directional transmission dynamics (e.g., VRE+MRSA → VRSA)
If the PDF does not display, click here to open it directly.
Abstract
Background
Prior long-term-care (LTC) studies confound anatomic reservoir and pathogen effects when multiple multidrug-resistant organisms (MDROs) circulate. We created a four-tier clearance framework to disentangle site- and species-specific behavior and quantify decolonization, mortality, and asymmetric co-colonization.
Methods
Between January 2024 and May 2025, 98 LTC residents colonized with carbapenem-resistant Enterobacterales (CRE), vancomycin-resistant Enterococcus (VRE), or multidrug-resistant Pseudomonas aeruginosa (MRPA) or Acinetobacter baumannii (MRAB) underwent weekly stool, urine, sputum, wound, blood cultures, yielding a total of 2,772 specimens. Clearance—operationally defined as three consecutive negatives—was analyzed across four tiers (site-atomic, pathogen-atomic, pathogen-level, patient-level). Conditional-probability tables were constructed to summarize how frequently the four MDROs co-colonized the same patient.
Results
Clearance was slowest in stool and for CRE, independent of patient attributes of age or sex. In this LTCF cohort, mortality depended on sputum carriage and host factors rather than pathogen identity. More than half of residents carried multiple MDROs, and conditional-probability analysis revealed asymmetric co-colonization: non-CRE organisms almost always co-colonized with CRE, whereas the reverse was uncommon.
Conclusions
The hierarchical analysis showed that, contrary to common expectations, decolonization was independent of patient attributes (age and sex) and that, in this LTCF setting, mortality was unrelated to pathogen identity. Asymmetric co-colonization therefore warrants automatic CRE screening following MRAB, MRPA, or VRE isolation, and underscores the need to investigate directional co-colonization patterns among multiple MDRO co-colonizations commonly observed in LTCFs—as exemplified by vanA transfer from VRE to MRSA (methicillin-resistant Staphylococcus aureus) yielding VRSA (vancomycin-resistant Staphylococcus aureus).
Introduction
Multidrug-resistant organisms (MDRO) infections pose a critical global health threat and are associated with worse outcomes than drug-susceptible infections [1]. Although most MDRO research has focused on acute-care or tertiary hospital settings, long-term care facilities (LTCF) are increasingly recognized as significant reservoirs of resistant pathogens [2]. Outcomes of MDRO infections vary by infection site, organism type, and the presence of polymicrobial infections [3, 4]. Co-colonization—where patients harbor multiple MDROs—is being reported frequently in acute and post-acute settings, further complicating infection control and worsening clinical outcomes [5].
Prior longitudinal studies of MDRO carriage exhibit one or more of the following limitations: single‐pathogen focus, retrospective design, limited sample sizes, short‐term study periods, heterogeneous follow-up intervals, and complex symptom-driven follow-up endpoints [6-11]. More critically, they seldom account for the intertwined effects of anatomical site and pathogen, and almost never examine the directional, asymmetric co‐colonization of multiple MDROs—leaving two critical gaps unaddressed. First, intra‐patient heterogeneity is obscured: some pathogens decolonize rapidly while others prolong quarantine [11], and different anatomical sites (e.g., sputum versus stool) exhibit markedly divergent decolonization kinetics for the same pathogen [12]. Second, the asymmetric nature of co‐colonization is overlooked: the presence of one pathogen often predicts carriage of another, yet the reverse association may be weak [13].
In the absence of a routinely recommended decolonization strategy for MDRO carriage, understanding clearance dynamics is critical, as infection-control policies in LTCFs must rely on natural clearance. Building on prior work and within existing clinical constraints, a prospective cohort study was conducted in a Korean LTCF, tracking a sizable resident population over an extended period during which hundreds of adjudicated events—clearance (operationally defined here as three consecutive negative cultures), death, or censoring—were captured and classified within a four-tier analytic hierarchy to minimize confounding by anatomical reservoir and pathogen. This four-tier framework, combined with conditional-probability analysis, provides a rigorous method for quantifying decolonization dynamics, mortality, and the directional co-colonization patterns that emerge when multiple MDROs circulate.
Methods
Study design and setting
This prospective cohort study of MDRO colonization dynamics was conducted from January 2024 through May 28, 2025, at Seoul Smart Convalescent Hospital (SSCH), a LTCF in the Republic of Korea. Patients were placed in single rooms whenever possible; if capacity was exceeded, cohort isolation was applied in multi-bed rooms. Adults aged ≥18 years were enrolled if they had colonization at baseline, defined as (i) an MDRO-positive culture detected on admission screening or during symptom-driven testing within the facility, or (ii) transfer from another hospital with documented MDRO carriage. Although SSCH routinely admits patients retrospectively identified as MDRO carriers at other hospitals for quarantine, all surveillance data in this study were collected prospectively after enrollment. “Clearance” was operationally defined, per Korea Disease Control and Prevention Agency (KDCA) guidelines, as three consecutive negative cultures—each obtained at least three days apart—from every anatomic site that had previously yielded a target organism; we used this definition as a clear endpoint for observing quarantined patients. Participants were followed until clearance, death, administrative censoring on May 28, 2025, or censoring at the date of the last weekly culture due to permanent discharge, transfer without return, or withdrawal of consent, whichever occurred first. Patient attributes (age and birth-assigned biological sex) and clinical endpoints, including in-hospital death, were extracted from the electronic medical record. The Institutional Review Board of SSCH—registered with the Korean Ministry of Health and Welfare (Registration No. 3-70094812-AB-N-01)—approved the study protocol (IRB No. 2024-CR-001; approved January 2, 2024), and all participants (or their legal guardians) provided written informed consent in accordance with the Declaration of Helsinki and Korean Good Clinical Practice guidelines.
Eligibility criteria and target organisms
Eligible participants were adult inpatients colonized with at least one of four prespecified MDROs—carbapenem-resistant Enterobacterales (CRE), vancomycin-resistant Enterococcus (VRE), multidrug-resistant Pseudomonas aeruginosa (MRPA), or multidrug-resistant Acinetobacter baumannii (MRAB), where KDCA mandates isolation exclusively for CRE and leaves isolation of other MDROs to institutional discretion. Residents whose only MDRO fell outside these four categories—such as carbapenem-resistant Pseudomonas aeruginosa (CRPA) or carbapenem-resistant Acinetobacter baumannii (CRAB)—were excluded because KDCA guidelines specify MRPA and MRAB, not CRPA or CRAB, for quarantine. Species were identified by MALDI-TOF MS, and antimicrobial susceptibility testing was performed with the VITEK-2 system; interpretations followed CLSI M100 breakpoints [14].
Surveillance culture protocol
Upon first documentation of MDRO carriage, residents were enrolled in a structured surveillance program. At baseline, rectal swabs and urine cultures were obtained to screen for CRE and VRE, and urine and sputum cultures with susceptibility testing were collected for all four target organisms. Thereafter, once an anatomic site tested positive for any target MDRO, we obtained weekly rectal swabs, urine specimens, and sputum specimens from that site. Cultures were collected at least once per week, even if a resident was briefly transferred to a nearby acute-care hospital for treatment or procedure and then readmitted; this ensured at least one culture per week. Missing a weekly culture constituted an exclusion criterion; specifically, patients who could not complete uninterrupted weekly surveillance—such as those briefly transferred and readmitted—were excluded. To allow peers to examine the full culture sequence, all results have been released in Supplementary Dataset S1 (RawDataset.zip) for complete transparency and potential secondary analysis. Rigorous compliance was enforced by national policy: the Korean Health Insurance Review and Assessment Service (HIRA) withholds reimbursement for isolation care if mandated cultures are skipped. Blood and wound specimens were obtained only when clinically indicated or when a referring facility had already reported a positive result. Stool cultures were omitted for MRPA and MRAB, as these non-fermenters rarely colonize the gastrointestinal tract and prior validation studies demonstrated negligible yield [15, 16]. Each isolate was logged by pathogen, anatomic site, and collection date.
Multi-Tiered Hierarchical Clearance Classification
The tiers progress from the most granular—site-atomic clearance (SAC) and pathogen-atomic clearance (PAC)—to pathogen-level clearance (PLC) and, ultimately, patient-level clearance (PtLC). Because SAC and PAC are novel terms, detailed plain-language definitions with an illustrative example are provided in Supplementary Note S2. When multiple MDROs are present, with potential conflation between site and pathogen, SAC reflects clearance per individual site, allowing comparisons across the atomic unit of reservoir sites (stool, urine, sputum, wound, blood), whereas PAC reflects clearance with respect to pathogens, allowing comparisons across the atomic unit of the pathogen (CRE, VRE, MRAB, MRPA).
SAC is achieved when a single anatomic site yields three consecutive negative cultures for the given pathogen previously isolated, regardless of culture results from other sites for the same pathogen or from any sites for other pathogens. PAC parallels SAC but shifts the focus to the organism: a PAC event is recorded when a specific pathogen produces three consecutive negative cultures at a given site, independent of its persistence at other sites or the coexistence of other organisms. PLC aggregates all sites within a single patient for one pathogen. It is achieved when every site that ever yielded that pathogen records three consecutive negative cultures, thereby documenting eradication of that specific pathogen from the host. PtLC sits atop the hierarchy and serves as the operational trigger for discontinuing transmission-based precautions. It requires three consecutive negative cultures from every site that has ever harbored any target MDRO in that patient. Although PLC and PtLC might seem similar, they serve distinct purposes: PLC defines eradication of a particular organism from all previously positive sites in a single patient, whereas PtLC extends this concept to the global eradication of every colonizing pathogen.
Statistical Analysis
All analyses were performed using the statistical software R, version 4.4.1 (the R Foundation for Statistical Computing, Vienna, Austria) on macOS. The primary endpoint was time to clearance, measured in days from the index positive culture to the first of three consecutive negative cultures meeting the study’s clearance criteria. The secondary endpoint was all-cause, in-hospital mortality, calculated from the date of the first post-admission culture. Mortality was evaluated only at the atomic strata—site-atomic death (SAD) script and pathogen-atomic death (PAD).
Kaplan–Meier curves were generated for each hierarchical tier to characterize colonization persistence and overall survival; medians and 95 % confidence intervals (CIs) were read directly from the curves [17]. When fewer than 50 % of subjects in a stratum experienced the event, the median was reported as “not reached” (NR); when a Greenwood‐based bound could not be estimated, it was reported as “not estimable” (NE). The effects of age (continuous) and sex on clearance and mortality were assessed with Cox proportional-hazards models, and results are presented as hazard ratios (HRs) with 95 % CIs. Two-sided P values < 0.05 were considered statistically significant.
Pathogen co-colonization was examined by converting the patient-by-pathogen presence–absence matrix into a conditional-probability matrix, P(b | a), representing the probability of isolating pathogen b at any time—preceding, concurrent with, or following—the isolation of pathogen a in the same patient.
Results
Study Population and Culture Episodes
From January 2024 through May 28, 2025, the study enrolled 124 patients (Fig. 1). Twenty-six patients were excluded according to the predefined criteria described in Methods. As a result, 98 patients were included in the analysis. The majority (n = 91, 92.9 %) were transferred from a nearby hospital for isolation, while seven were identified as MDRO carriers at SSCH itself. The median age was 79.5 years (IQR, 69.0–84.0), and 50 (51.0 %) were male. Across this cohort, a total of 2,772 culture episodes were recorded: 1,776 for CRE, 632 for VRE, 133 for MRAB, and 181 for MRPA.
Site-atomic clearance (SAC)
Ninety‐eight LTCF residents generated 274 SAC episodes: stool (n = 115), urine (n = 84), sputum (n = 63), wound (n = 11), and blood (n = 2) (Table 1). Blood is shown for completeness but was excluded from modeling because no clearances or deaths occurred. Kaplan–Meier curves differed significantly across sites (Fig. 2a; log‐rank P < 0.001). Stool was the most persistent reservoir (median time‐to‐clearance, 181d; 95% CI, 134–273), whereas sputum, urine, and wound colonization cleared in median 78d (95% CI, 44–112), 47d (95% CI, 36–82), and 28d (95% CI, 21–NE [upper bound not estimable]), respectively. In a multivariable Cox model using stool as the reference (Table 2), the adjusted clearance rate ratio increased 2.66-fold for sputum (hazard ratio [HR], 2.66; 95% CI, 1.60–4.44), 2.84-fold for urine (HR, 2.84; 95% CI, 1.83–4.40), and 5.25-fold for wounds (HR, 5.25; 95% CI, 2.50–10.99); neither age (HR, 0.999; 95% CI, 0.984–1.014) nor sex (male HR, 1.16; 95% CI, 0.80–1.67) was independently associated with clearance. At the SAC tier, stool exhibited the slowest clearance with marked variation across sites, and clearance was independent of patient age and sex.
Site-atomic death (SAD)
During the same interval, 88 of the 274 site-atomic episodes ended in death (Fig. 2b). Median survival differed significantly by anatomical site (log-rank P = 0.02): it was shortest for sputum-colonized residents (median, 68d; 95% CI, 51–NE), intermediate for stool (144d; 95% CI, 93–244), and longest for urine (189d; 95% CI, 155–NE); wound and blood episodes were too sparse for reliable estimates. In the fully adjusted Cox model using sputum as the reference (Table 2), stool carriage was associated with a 41% lower mortality hazard (HR, 0.59; 95% CI, 0.36–0.97) and urine carriage with a 51% lower hazard (HR, 0.49; 95% CI, 0.26–0.92); the wound–sputum contrast did not reach statistical significance (HR, 0.42; 95% CI, 0.05–3.14). Each additional year of age increased the risk of death by 4.4% (HR, 1.044; 95% CI, 1.022–1.067), and male sex conferred a 90% excess hazard (HR, 1.91; 95% CI, 1.23–2.96). At the SAD tier, mortality was highest in sputum-colonized residents, varied significantly by site, and—unlike clearance—was influenced by age and sex.
Pathogen-atomic clearance (PAC)
All 274 PAC episodes—CRE (n = 156), VRE (n = 68), MRPA (n = 29), and MRAB (n = 21)—were evaluable (Table 1). Kaplan–Meier curves separated sharply by pathogen (Fig. 2c; log-rank P ≈ 1 × 10⁻⁹). CRE was the most tenacious organism, with a median time-to-clearance of 159d (95% CI, 111–246) and a crude clearance rate of 32.1%. In contrast, MRAB cleared in a median 28d (95% CI, 26–NE) with a 76.2% clearance rate; MRPA cleared in 27d (95% CI, 24–94) with a 69.0% rate; and VRE cleared in 61d (95% CI, 51–139) with a 51.5% rate. In a multivariable Cox model using CRE as the reference (Table 2), the adjusted clearance rate ratio was 5.30-fold higher for MRAB (HR, 5.30; 95% CI, 2.96–9.51), 5.12-fold higher for MRPA (HR, 5.12; 95% CI, 3.00–8.75), and 2.04-fold higher for VRE (HR, 2.04; 95% CI, 1.32–3.16); neither age (HR, 0.9965; 95% CI, 0.981–1.012) nor male sex (HR, 1.09; 95% CI, 0.76–1.58) was significant. At the PAC tier, CRE cleared most slowly, VRE intermediately, and MRAB/MRPA cleared fastest; as at the SAC tier, clearance was independent of patient age and sex.
Pathogen-atomic death (PAD)
Of the 274 pathogen-atomic episodes, 88 ended in death (Fig. 2d; log-rank P = 0.80), and the survival curves for CRE, VRE, MRAB, and MRPA were virtually identical. Median survival was 136d (95% CI, 102–189) for CRE and 293d (95% CI, 89–NE) for VRE; medians for MRAB and MRPA were not reached (lower 95% CI bound NE for MRAB and 41 d for MRPA). In a fully adjusted Cox model (Table 2), none of the non-CRE pathogens—MRAB (HR, 0.98; 95% CI, 0.30–3.20), MRPA (HR, 0.90; 95% CI, 0.36–2.27), or VRE (HR, 0.82; 95% CI, 0.48–1.41)—significantly affected mortality risk compared with CRE. Instead, host factors predominated: each additional year of age increased the mortality hazard by 4.8% (HR, 1.048; 95% CI, 1.025–1.071), and male sex conferred a 92% excess hazard (HR, 1.92; 95% CI, 1.24–2.98). At the PAD tier, no specific organism uniquely predicted death in our LTCF cohort; instead, mortality was driven by host factors (age and sex). Notably, clearance at the SAC and PAC tiers was independent of those patient attributes.
Pathogen-level clearance (PLC)
The cohort generated 173 PLC episodes—CRE (n = 85), VRE (n = 44), MRPA (n = 23), and MRAB (n = 21)—of which 14/85 (16.5%), 17/44 (38.6%), 16/23 (69.6%), and 14/21 (66.7%) met the clearance definition, respectively (Table 1). Median time-to-clearance was 68 d (95% CI, 53–246) for CRE, 57d (95% CI, 41–139) for VRE, 26d (95% CI, 21–94) for MRPA, and 28d (95% CI, 27–78) for MRAB. Kaplan–Meier curves appeared broadly similar (Fig. 2e), and the global log-rank test indicated modest differences among pathogens (P = 0.0397). Thus, unlike the PAC tier—where clearance kinetics varied sharply by species (log-rank P ≈ 1 × 10⁻⁹)—aggregation at the PLC tier attenuates but does not entirely eliminate those differences, as reflected by the more modest global log-rank P value of 0.0397, which only just meets the conventional 0.05 threshold.
Patient-level clearance (PtLC)
Complete PtLC remained uncommon: 18 events occurred among 17 of 98 residents (18.4%), who achieved three consecutive negative cultures at all previously positive sites (Table 1). As shown in Fig. 1, one resident (patient 1004) reached PtLC twice—first after multi-MDRO clearance and again following re-isolation. The overall median time to PtLC was 97 d (95 % CI, 53–238; Fig. 2f), exceeding the pathogen-level medians for CRE (68 d), VRE (57 d), MRPA (26 d), and MRAB (28 d), as PtLC incorporates clearance across all four MDROs and thus cannot be shorter than any individual PLC.
Co-colonization profile
The upper panel of Table 3 shows patient counts. Among the 98 residents, CRE remained the dominant organism, colonizing 80 individuals (81.6%), whereas VRE, MRAB, and MRPA were present in 42.9%, 17.3%, and 19.4%, respectively. Co-carriage was common: 49 residents (50.0%) harbored at least two target MDROs, and 18 (18.4%) carried three or more, underscoring the polymicrobial pressure typical of long-term care. The most frequent dyad was CRE + VRE (27 of 98, 27.6%), followed by CRE + MRPA (16 of 98, 16.3%) and CRE + MRAB (13 of 98, 13.3%); no other pair exceeded 10% prevalence.
Notably, the lower panel of Table 3 presents a strongly asymmetric co-colonization network centered on CRE: 76.5 % of MRAB carriers and 84.2 % of MRPA carriers were also colonized with CRE, whereas the reverse probabilities were only 16.2 % and 20.0 %, respectively. For transparency, patient-level co-colonization profiles are provided in Supplementary Table S3.
Discussion
The present study refines our understanding of colonization dynamics in LTCFs by introducing a four-tier hierarchical framework—SAC, PAC, PLC, and PtLC—to disentangle clearance kinetics. At the PLC tier, organism‐specific differences in clearance are substantially attenuated but not eliminated (log-rank P = 0.0397), contrasting sharply with the highly significant differences observed at the PAC tier (log-rank P ≈ 1 × 10⁻⁹) and confirming that this additional stratification mitigates reservoir-driven confounding. This occurs because PLC has an intrinsic limitation: by aggregating sites and pathogens, it requires every previously culture-positive site to register three consecutive negative cultures. For example, CRE’s PLC can be substantially delayed by persistent stool colonization, even though sputum colonization clears much more quickly. To address this confounding, the PAC and SAC tiers were devised to focus separately on pathogen and site, respectively. The PAC tier captures intrinsic species behavior across sites, showing that some organisms (e.g., VRE) clear rapidly regardless of location, whereas others (e.g., CRE) persist. Prior reports confirm this pattern: 87.5% of VRE carriers had cleared compared with only 50% of CRE carriers [18], median ~26 weeks for VRE [19], while ~65% of CRE carriers remained colonized at one year [20]. These differences likely reflect ecological factors, as CRE persist within the colonic mucus layer whereas VRE are excluded [21]. The SAC tier provides a site-specific perspective: stool cultures clear extraordinarily slowly, reflecting the gut’s role as a protected reservoir where MDROs persist [6, 8], whereas sputum and urine often convert quickly because non-GI sites are more responsive to source control and local interventions [9, 10]—contrasts that PLC or PtLC would mask. For example, in patients with IDs 1023 and 1076, stool clearance was markedly delayed, while multiple urine sites cleared rapidly.
One might be tempted to attribute mortality to the colonizing pathogen, but when the intertwined confounding effects of anatomic site and species are disentangled, this assumption does not hold in an LTCF setting. PAD-tier survival curves for CRE, VRE, MRAB, and MRPA were nearly superimposable (log-rank P = 0.80; Fig. 2d), suggesting that no organism carried a significantly different mortality risk. This finding is plausible because LTCF residents are often chronically colonized with MDROs or merely isolated and quarantined without overt infection symptoms, meaning these pathogens contribute little to mortality. These results underscore that, in LTCFs, although organism-centered measures remain critical for transmission prevention and mortality prediction, improving survival hinges on addressing host factors (advanced age and male sex) and site-specific vulnerabilities—particularly sputum carriage—rather than solely targeting individual pathogens.
Patient attributes—age and sex—were significant predictors of mortality at both the SAD and PAD tiers. Although one might intuitively link age and sex to clearance, our study demonstrates the opposite: at both the SAC and PAC tiers, clearance was independent of these patient attributes, a distinction that can inform discussions with patients and caregivers—for example, those who wonder whether their elderly parent can be decolonized. This likely reflects that clearance is driven by local microbiological and environmental dynamics—biofilm architecture, microbiome composition, antibiotic pressure, and tissue comorbidities [8, 22, 23]—none of which correspond closely to systemic immunosenescence or sex-based immune differences.
This study has several limitations. First, our clearance definition was calibrated to Korean national regulations, which may limit generalizability to other settings. Second, follow-up began with the first positive culture at our facility, which may not represent the true onset of colonization; some residents were already colonized before admission, so carriage duration may have been underestimated. Third, universal baseline screening from negative through acquisition to clearance was not performed and was impractical in our LTCF setting: in Korea, isolation care is reimbursed fee-for-service, while non-isolation long-term care is largely reimbursed under a bundled payment system. Once acquisition is documented, caregivers expect serial cultures to enable isolation release, but they generally resist pre-acquisition screening because it could trigger quarantine, and hospitals must absorb the cost under bundled payment models. There is limited incentive on either side, making universal surveillance infeasible.
In this real-world context, automatic CRE screening after MRAB, MRPA, or VRE serves as a pragmatic alternative to universal surveillance. As shown in Table 3, co-colonization was strongly asymmetric. Because our conditional probabilities are longitudinal rather than contemporaneous at the trigger event, they do not themselves yield a cross-sectional number-needed-to-test (NNT). Nonetheless, more than three-quarters of MRAB carriers (76.5%) and over four-fifths of MRPA carriers (84.2%) also harbored CRE, whereas only 16.2% of CRE carriers had MRAB and 20.0% had MRPA. For facilities without active surveillance, this translates into targeted CRE screening with a single batched culture run for the flagged subset, reducing both nursing effort and laboratory costs compared with universal screening. Beyond this practical application, it remains essential to investigate directional dynamics more broadly: horizontal transfer of vanA from VRE to MRSA has produced de novo VRSA in co-colonized patients [24]. Such cases demonstrate how simultaneous colonization within the same niche facilitates gene transfer, underscoring the directional and asymmetric nature of these interactions.
In conclusion, this study offers a distinctive contribution by pairing a four-tier hierarchical framework with conditional-probability mapping to disentangle the often-confounded influences of pathogen and anatomic site in LTCFs—an angle rarely explored in previous MDRO research. It overturns intuitive expectations by showing that, in this LTCF, mortality depends more on colonization site than on pathogen identity (MDROs here are often detected as chronic colonizers rather than causing acute illness), whereas clearance is independent of patient attributes such as age and sex—a finding that may reassure caregivers worried that advanced age impedes decolonization. The asymmetric co-colonization pattern we uncovered—non-CRE organisms almost always co-colonized with CRE, whereas CRE carriers rarely host other MDROs—supports automatic CRE screening whenever MRPA, MRAB, or VRE is detected and further highlights the need for systematic investigation of directional co-colonization dynamics—such as transfer of vanA from VRE to MRSA leading to de novo VRSA, thereby increasing P(VRSA | VRE + MRSA)—in LTCFs burdened by multiple MDROs.
References
[1] EclinicalMedicine: Antimicrobial resistance: a top ten global public health threat. EClinicalMedicine 2021;41: 101221.
[2] O'Fallon E, Pop-Vicas A, D'Agata E: The emerging threat of multidrug-resistant gram-negative organisms in long-term care facilities. J Gerontol A Biol Sci Med Sci 2009;64(1): 138-141.
[3] Centers for Disease C, Prevention: Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morbidity and mortality weekly report 2013;62(9): 165-170.
[4] Cilloniz C, Calabretta D, Palomeque A, Gabarrus A, Ferrer M, Marcos MA, et al.: Risk Factors and Outcomes Associated With Polymicrobial Infection in Community-Acquired Pneumonia. Arch Bronconeumol 2025.
[5] Tadese BK, DeSantis SM, Mgbere O, Fujimoto K, Darkoh C: Clinical Outcomes Associated with Co-infection of Carbapenem-Resistant Enterobacterales and other Multidrug-Resistant Organisms. Infect Prev Pract 2022;4(4): 100255.
[6] Mo Y, Hernandez-Koutoucheva A, Musicha P, Bertrand D, Lye D, Ng OT, et al.: Duration of Carbapenemase-Producing Enterobacteriaceae Carriage in Hospital Patients. Emerg Infect Dis 2020;26(9): 2182-2185.
[7] Nutman A, Lerner A, Fallach N, Schwartz D, Carmeli Y: Likelihood of persistent carriage of carbapenem-resistant Acinetobacter baumannii on readmission in previously identified carriers. Infect Control Hosp Epidemiol 2019;40(10): 1188-1190.
[8] Byers KE, Anglim AM, Anneski CJ, Farr BM: Duration of colonization with vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23(4): 207-211.
[9] Haverkate MR, Derde LP, Brun-Buisson C, Bonten MJ, Bootsma MC: Duration of colonization with antimicrobial-resistant bacteria after ICU discharge. Intensive Care Med 2014;40(4): 564-571.
[10] Pacio GA, Visintainer P, Maguire G, Wormser GP, Raffalli J, Montecalvo MA: Natural history of colonization with vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, and resistant gram-negative bacilli among long-term-care facility residents. Infect Control Hosp Epidemiol 2003;24(4): 246-250.
[11] Lin I-W, Huang C-Y, Pan S-C, Chen Y-C, Li C-M: Duration of colonization with and risk factors for prolonged carriage of multidrug resistant organisms among residents in long-term care facilities. Antimicrobial Resistance & Infection Control 2017;6.
[12] Zirakzadeh A, Patel R: Vancomycin-resistant enterococci: colonization, infection, detection, and treatment. Mayo Clin Proc 2006;81(4): 529-536.
[13] Heinze K, Kabeto M, Martin ET, Cassone M, Hicks L, Mody L: Predictors of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci co-colonization among nursing facility patients. Am J Infect Control 2019;47(4): 415-420.
[14] Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA. 2023.
[15] Lortholary O, Fagon JY, Buu Hoi A, Mahieu G, Gutmann L: Colonization by Acinetobacter baumanii in intensive-care-unit patients. Infect Control Hosp Epidemiol 1998;19(3): 188-190.
[16] Estepa V, Rojo-Bezares B, Torres C, Saenz Y: Faecal carriage of Pseudomonas aeruginosa in healthy humans: antimicrobial susceptibility and global genetic lineages. FEMS Microbiol Ecol 2014;89(1): 15-19.
[17] Kleinbaum DG, Klein M. Survival Analysis: A self-Learning Text. LLC, 233 Spring Street, New York, NY 10013, USA: Springer Science + Business Media. 2012.
[18] Dinh A, Fessi H, Duran C, Batista R, Michelon H, Bouchand F, et al.: Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect 2018;99(4): 481-486.
[19] Shenoy ES, Paras ML, Noubary F, Walensky RP, Hooper DC: Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC Infect Dis 2014;14: 177.
[20] Bar-Yoseph H, Hussein K, Braun E, Paul M: Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: systematic review and meta-analysis. J Antimicrob Chemother 2016;71(10): 2729-2739.
[21] Caballero S, Carter R, Ke X, Susac B, Leiner IM, Kim GJ, et al.: Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant Enterococcus faecium and Carbapenem-Resistant Klebsiella pneumoniae. PLoS Pathog 2015;11(9): e1005132.
[22] Ciobotaro P, Flaks-Manov N, Oved M, Schattner A, Hoshen M, Ben-Yosef E, et al.: Predictors of Persistent Carbapenem-Resistant Enterobacteriaceae Carriage upon Readmission and Score Development. Infect Control Hosp Epidemiol 2016;37(2): 188-196.
[23] Seong H, Lee SK, Cheon JH, Yong DE, Koh H, Kang YK, et al.: Fecal Microbiota Transplantation for multidrug-resistant organism: Efficacy and Response prediction. J Infect 2020;81(5): 719-725.
[24] Marchaim D, Perez F, Lee J, Bheemreddy S, Hujer AM, Rudin S, et al.: "Swimming in resistance": Co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa. Am J Infect Control 2012;40(9): 830-835.
© 2025 King Saud Bin Abdulaziz University for Health Sciences. Licensed under CC BY 4.0.